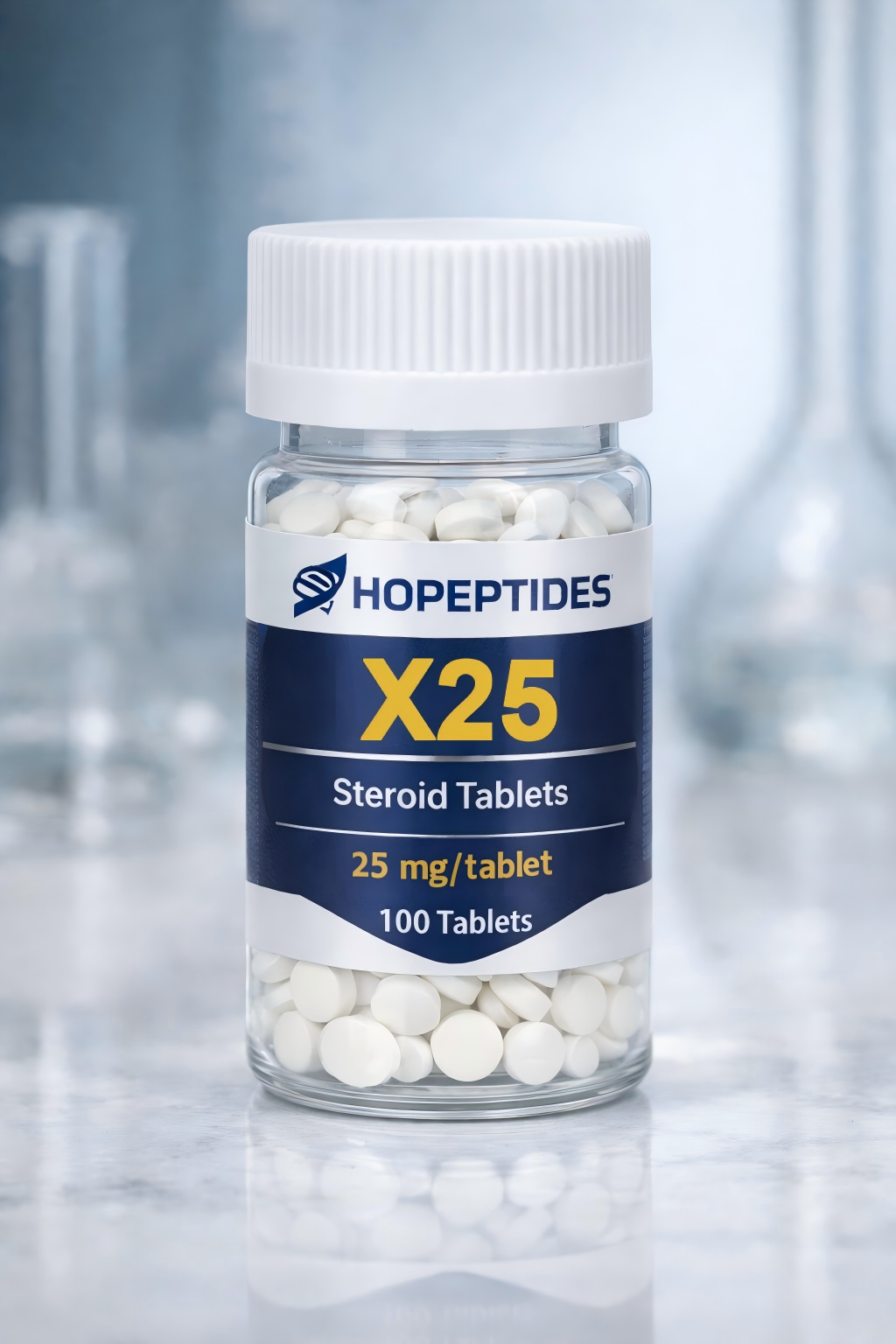
ANAVAR X25 (Oxandrolone 25 mg Tablets) – Research Use Only
ANAVAR X25 is a synthetic small-molecule research compound containing Oxandrolone at a concentration of 25 mg per tablet. This product is intended exclusively for laboratory, analytical, and preclinical research applications.
The tablet format provides consistent dosing, handling convenience, and inventory control, making it suitable for research supply, wholesale distribution, and private-label applications.
Key Product Information
-
Product Name: ANAVAR X25
-
Active Compound: Oxandrolone
-
Strength: 25 mg per tablet
-
Form: Oral solid tablet (research format)
-
Appearance: White to off-white tablets
-
Packaging: Secure, tamper-resistant bulk or multi-unit packaging
Research Applications
ANAVAR X25 is commonly referenced in non-clinical research settings for:
-
Analytical reference and compound identification
-
Small-molecule metabolic and anabolic pathway research
-
Tablet stability and formulation studies
-
Laboratory catalog distribution and private-label research supply
All downstream use must remain non-clinical and non-therapeutic.
Manufacturing & Quality Assurance
-
Manufactured under controlled laboratory conditions
-
Emphasis on tablet weight consistency and purity
-
Batch-to-batch consistency with traceable documentation
-
COA verification available for all batches
Storage & Handling
-
Store in a cool, dry place, away from light and moisture
-
Handle using standard laboratory safety procedures
-
Avoid ingestion, inhalation, or contact with skin
Wholesale / Distributor Advantages
-
Uniform tablet format for ease of inventory and resale
-
COA-supported batch verification ensures research confidence
-
Neutral wording suitable for private-label branding
-
Reliable supply for repeat wholesale orders
Regulatory & Compliance Notice
This product is intended for research and laboratory use only.
Not for human consumption, animal use, medical use, diagnostic use, or therapeutic application.
This product has not been evaluated or approved by the U.S. Food and Drug Administration (FDA).




Reviews
There are no reviews yet.